ISPE is a global industry leader in scientific, technical, and regulatory advancement throughout the entire pharmaceutical lifecycle. As a result, ISPE is in a strong position and available to assist the US government, its allies, and like-minded regulatory partners with implementation of recommendations emerging from a report, Building Resilient Supply Chains, Revitalizing American Manufacturing, and Fostering Broad-Based Growth: 100-Day Reviews. These reviews were commissioned by the US Administration examining “America’s Supply Chains” in four strategically important areas one of which was pharmaceuticals and active pharmaceutical ingredients (APIs).
The COVID-19 pandemic and resulting economic dislocation as well as other extreme weather events revealed long-standing vulnerabilities in US supply chains. The resulting report explores in great depth the causes of these vulnerabilities and concludes there are a set of inter-related themes and findings that contribute to supply chain vulnerabilities. These are:
- Insufficient US manufacturing capacity
- Misaligned Incentives and short-termism in private markets
- Industrial Policies Adopted by Allied, Partner, and Competitor Nations
- Geographic concentration in global sourcing
- Limited International Coordination
In response to a request from a US federal agency, ISPE, in keeping with its vision and mission and to provide solutions to complex pharmaceutical industry challenges and enhance efforts to develop, manufacture, and reliably deliver quality medicines to patients, produced a relevant, background report, Increasing Domestic Resiliency in the Supply of Essential Active Pharmaceutical Ingredients, which was issued in December 2020. This report was developed by 14 subject matter experts and was available as a resource for the pharmaceuticals and API task team working on the 100-Day Reviews report.
The 100-Day Reviews report highlights the following two priority objectives for improvements related to the reliability of the pharmaceutical and API supply chains:
- Improve supply chain transparency and incentivize resilience
- Increase the economic sustainability of US and allied drug manufacturing and distribution.
ISPE has a series of initiatives and working groups containing very knowledgeable subject matter experts and they are producing material such as best practice guidance documents, informational webinars, presentations, and articles highly relevant to detailed recommendations given in the pharmaceuticals and API section of the 100-Day Reviews report. A summary of these teams is given in the following figure.

DRUG SHORTAGES
Almost the whole focus of the 100-Day Reviews report is related to the challenge of identifying the root causes of drug shortages and mitigating identified risks.
ISPE has had a team working on this topic since 2013. A series of reports and articles has been published to assist industry to analyze potential causes of drug shortages and to introduce programs and procedures to mitigate the risks to supply continuity. Some key examples are:
- The ISPE Drug Shortages Prevention Plan, 2014 – an actionable plan intended to help the pharmaceutical and biopharmaceutical industry avoid drug shortages that result from manufacturing and quality issues.
- The ISPE Drug Shortage Assessment & Prevention Tool, 2015 – provides manufacturers across the spectrum of the bio/pharmaceutical industry with methods to locate current and future inconsistencies across the pharmaceutical manufacturing supply chain. By using this tool, manufacturers will mitigate problems before they arise allowing them to provide an uninterrupted supply of safe, quality medicines to patients worldwide. The concept of maturity assessment was introduced for each of six dimensions of:
- Corporate culture
- Robust quality system
- Metrics
- Business continuity planning
- Communication with authorities
- Building capability
- Business Continuity Planning to Prevent Drug Shortages article, 2021 – introduces concepts for business continuity planning that:
- Are a multi-functional activity
- Should be rigorous for priority products
- Should evaluate both product specific risk and business/operational risks
- Should balance stockpiling/redundancy with agility
- Are not a one-time activity
- May benefit by health authority interactions and feedback
ISPE’s Drug Shortages team is currently finalizing a benchmarking study, launched in May 2021, which will be used to assess best practices and industry accomplishments in the dimension of business continuity planning to prevent drug shortages. The team will use the anonymized benchmarking study outcomes to prioritize and develop activities to further support the industry with improving business continuity planning.
There is an upcoming webinar, Opportunities for Drug Shortage Prevention – Industry and Regulator Views on Thursday, 30 September 2021, which is particularly relevant to the 100-Day Reviews report. In addition to representatives from the US FDA, regulators from France and Brazil will present as well as a representative from WHO. This webinar will:
- Provide the audience with a better understanding of the key opportunities to advance drug shortage prevention, particularly in the areas of business continuity planning and health authority engagement.
- Hear and explore current thinking on drug shortage prevention from global health authority regulators
- Share information on key resources available for improving drug shortage prevention and inspire companies to pursue improvements.
Relevant to the 100-Day Reviews report section “Explore the Creation/Expansion of a Virtual Strategic Stockpile of API Reserve and Other Critical Materials Managed by the Strategic National Stockpile, Including Finished Doses”, ISPE has a Blockchain Special Interest Group, which is examining the application of blockchain technology to assist with understanding the challenges to creating virtual stockpiling.
ISPE has continued working in all six dimensions identified in the Drug Shortage Assessment & Prevention Tool. Another good example is ISPE’s Advancing Pharmaceutical Quality (APQ) program, which is discussed below under the Quality Maturity heading.
CREATE QUALITY TRANSPARENCY
Quality Maturity
The 100-Day Reviews report contains the following references to quality management and quality maturity:
“ ………….working in cooperation with partners and allies is to create robust and mature quality management to ensure consistent and reliable drug manufacturing and quality performance. In its current form, the pharmaceutical marketplace does not recognize or reward investment in mature quality management.”
“HHS and FDA propose to create a program with a rating system aimed at recognizing and rewarding manufacturers for mature quality systems …….”
Since 2018 ISPE has been working on a quality maturity program called the Advancing Pharmaceutical Quality (APQ) Program, which is based on the ICH Q10, Pharmaceutical Quality System. It is an industry-for-industry program, which can help companies, sites, or departments understand their level of quality maturity and improve it, and which may help organizations prepare for any quality management rating system proposed by FDA or other regulatory agencies.
The goals, benefits, and principles set out in 2018 have remained unchanged, with the overarching goals of the APQ Program being to:
- Integrate quality management maturity, cultural, and operational excellence principles, tools, and approaches.
- Foster industry ownership of quality beyond compliance.
- Promote effective and efficient use of resources.
- Support and incentivize continual improvement.
- Encourage self-improvement and supplier improvement.
- Enable structured benchmarking, knowledge sharing, and learning among companies.
- Increase the reliability of supply for quality products.
- Offer routes for delivering a sustainable competitive advantage.
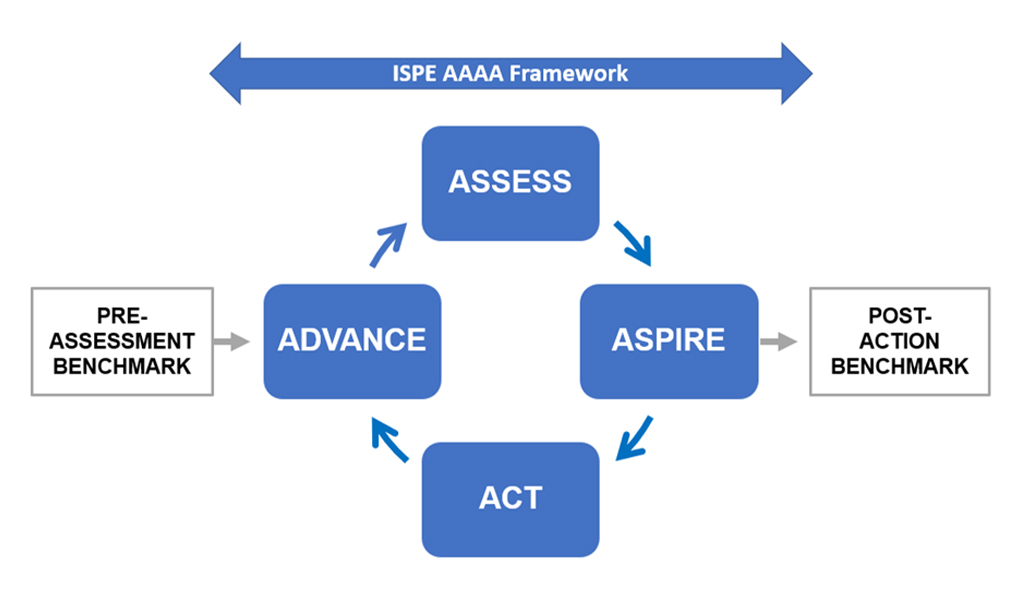
The APQ Program has been designed with an assess, aspire, act, and advance (AAAA) framework as a core to provide formal continual improvement opportunities. Assessment allows a baseline to be established and opportunities for potential continual improvement to be identified; aspire involves selecting and prioritizing the improvements to act upon; act requires a detailed, resourced action plan to be developed with targeted improvement outcomes; and advance evaluates and confirms the required outcomes have been achieved.
The APQ Program containing the AAAA framework is given in the following diagram.

The APQ AAAA framework is:
- A self-assessment process and toolkit
- Composed of four distinct but interconnected stages
- Based on a five-step maturity model
- Intended as an iterative continual improvement process
- A detailed quantitative and qualitative case study with criteria to evaluate the current state of quality, diagnose gaps, and identify improvement opportunities
To provide a quantitative baseline, an OPEX bench marking tool developed by the University of St. Gallen is included as a pre-and post-benchmarking exercise for the APQ AAAA framework, as shown in the above figure. This benchmarking could be performed optionally by St. Gallen or by self-application using tools provided by St. Gallen in the APQ Guide.
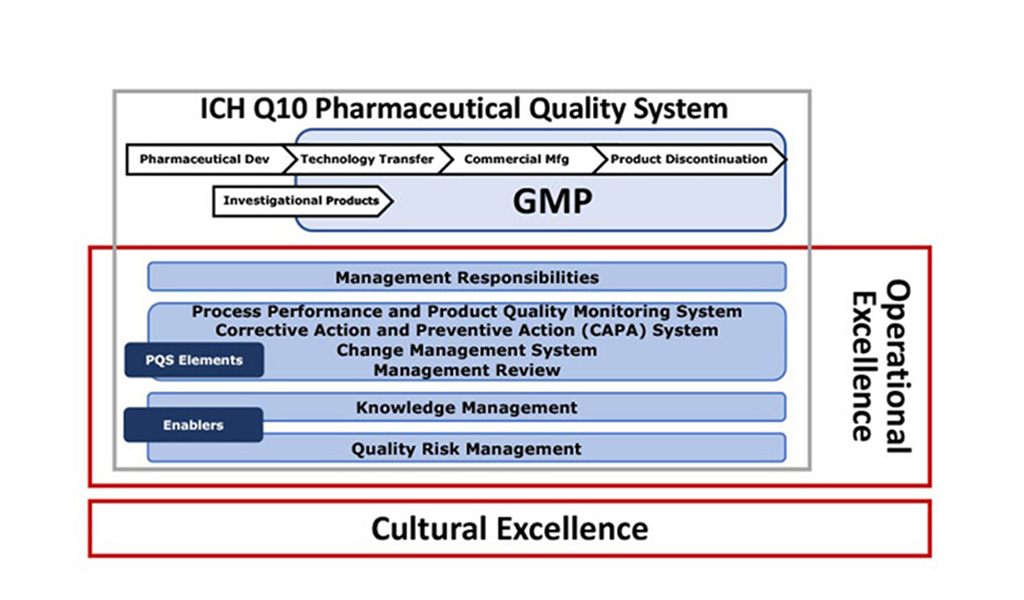
The APQ Program will consist of five Guides based on four ICH Q10, pharmaceutical quality system (PQS) elements;
- Corrective Action and Preventive Action (CAPA) system
- Management Review and Management Responsibilities
- Change Management system
- Process Performance and Product Quality Monitoring system
The following figure shows the four Guides in the center, with supporting body of knowledge.
The Corrective Action and Preventive Action (CAPA) and Management Review and Management Responsibilities Guides have been published. The Change Management Guide is scheduled for publication before the end of 2021 and the Process Performance and Product Quality Monitoring Guide will be published in 2022.
Additionally, the ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry has recently been published.
The figure below founded on ICH Q10 Annex 2 PQS Model shows how the APQ Program and its Guides complement ICH Q10.
INVEST IN RESEARCH AND DEVELOPMENT
Establish Novel Platform Production Technologies as Mainstream
The Review discusses “….. promoting R&D that develops innovative manufacturing processes and production technologies that will strengthen supply chain resilience.”
For nearly two decades, ISPE’s Product Quality Lifecycle (PQLI)® initiative has worked at the nexus of pharmaceutical manufacturing technology, and regulation to bring forward solutions that help advance new regulatory and technology approaches. A recent article summarizes the historical and current PQLI work in realizing this mission, thus supporting medicines reaching patients around the globe. Two PQLI working groups are very active in fields relevant to technologies highlighted in the 100-Day Reviews report review, namely:
- Continuous Manufacturing Working Group
- Transportable and Point of Care (POC) Working Group
In addition, ISPE recognizes that to support introduction of new technology, investment in training new people is required. Consequently, as part of the ISPE Foundation, ISPE has established a Workforce of the Future program sponsored by senior leaders from many multi-national pharmaceutical companies.
PROMOTE INTERNATIONAL COOPERATION AND PARTNER WITH ALLIES
A consistent feature of the 100-Day Reviews report is the need for the US to work with allies and like-minded partners.
ISPE has a strong international presence with many international conferences and meetings held in locations and in cooperation with regulatory agencies which could be considered strong allies and partners of the US. Examples are many, however, a recent relevant example is the Global Pharmaceutical Regulatory Summit held on 28 April 2021, entitled Distant Assessments, Audits, & Regulatory Guidance. The Summit, held virtually, brought together 11 regulators from different parts of the world to discuss how their approaches to GMP inspections have adapted to the COVID-19 pandemic. These regulators represented eight countries, the European Union, and World Health Organization (WHO).
An upcoming Drug Shortages webinar is yet another example of ISPE facilitating international discussion of a topic relevant to the 100-Day Review report.
CONCLUSION
ISPE is in a strong position and available to assist the US government, its allies, and like-minded regulatory partners with implementation of recommendations emerging from the US government 100-Day Reviews report. As an example, ISPE and its Drug Shortages Initiative team of industry experts is prepared to provide platforms for discussion with appropriate government or regulatory agencies in support of global harmonization activities regarding defining “essential” medicines that would be beneficial to the industry and the patients they serve.


